Isolated ocular Stevens-Johnson syndrome – An unusual case of cicatricial conjunctivitis
Patient Presentation
A 15 year old boy was referred by his gastroenterologist for a new “blistering conjunctivitis” in both eyes and blurring of vision over 4 weeks. His ocular history was notable for right amblyopia and he had a past medical history of acne and ulcerative colitis, for which he was on prednisolone 5mg daily, infliximab and mesalazine.
On presentation to the paediatric eye clinic, the patient complained of painful, red eyes with epiphora and reduced vision. Visual acuity was 6/15 unaided (no improvement with pinhole – NIPH) in the right eye and 6/9 unaided (6/6 with pinhole) in the left eye. Intraocular pressure was 17mmHg in the right eye and 18mmHg in the left eye. Refraction of the right eye was +0.25/+1.25 x 81° and the left eye was -9.00/+6.00 × 87°. Central corneal thickness was 547µm in the right eye and 537µm in the left eye with ultrasound pachymetry. Significant meibomian gland dysfunction, worse in the left eye, was noted and lid oedema was causing a mechanical ptosis. There was significant bilateral conjunctival inflammation and the left lower lid tarsal conjunctiva had a one-centimetre area of ulceration with fluorescein uptake. Prominent corneal nerves and moderate superficial punctate epithelial erosions were noted on both corneas but there was no corneal ulceration. The anterior chamber was deep and quiet and the lens was clear. Dilated fundal exam was normal. Systems review was notable for gastrointestinal distress and altered bowel habits. No urinary or respiratory symptoms were present and there was no associated joint pain or rash. There was no history of oral, upper respiratory tract or genital ulceration. Viral (HSV1/2, VZV, Adenovirus) and bacterial swabs were taken and the patient was started on fucithalmic acid ointment (three times daily), preservative free dexamethasone 0.1% (Dexafree) three times daily and regular lubricants.
On review, 3 weeks later, the patient reported worsening symptoms. The visual acuity reduced to 6/24 (6/18 with pinhole in the right eye) and 6/12 (NIPH) in the left eye. Small central corneal epithelial defects were present in both eyes. The cicatricial conjunctivitis had worsened with bilateral forniceal shortening noted. All swab reports were negative. At this point, Dexafree was increased to 2-hourly and the patient was referred to a specialist corneal clinic.
Diagnostic Testing
Anterior segment photos were taken in the cornea clinic which are shown below (Figure 1 & 2). These show diffusely injected tarsal and bulbar conjunctival surfaces bilaterally with forniceal shortening.
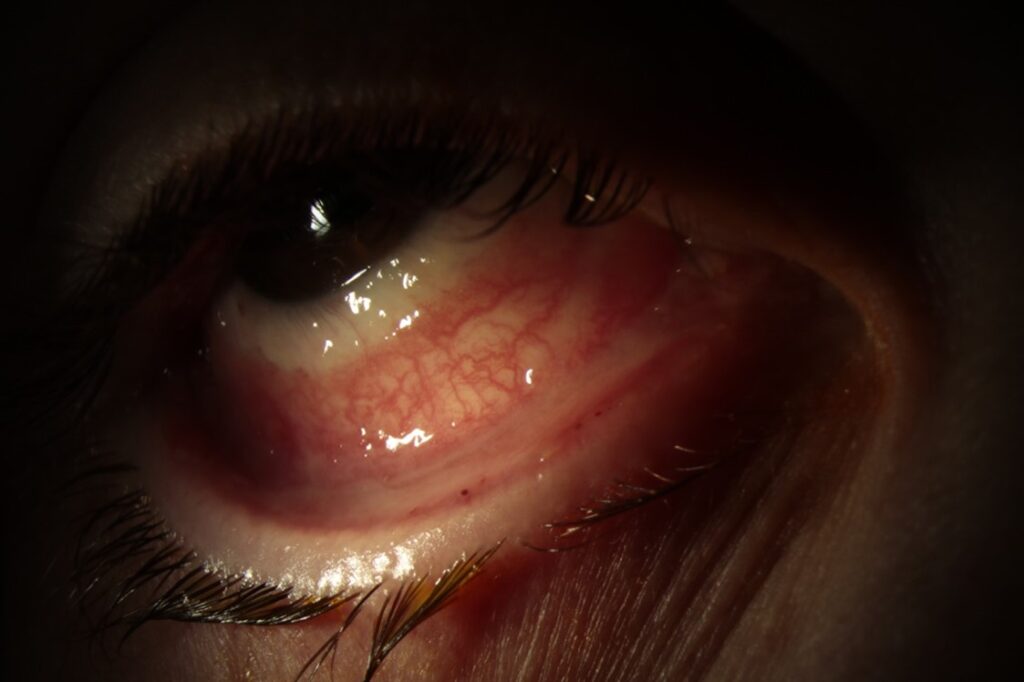
Figure 1. Right eye
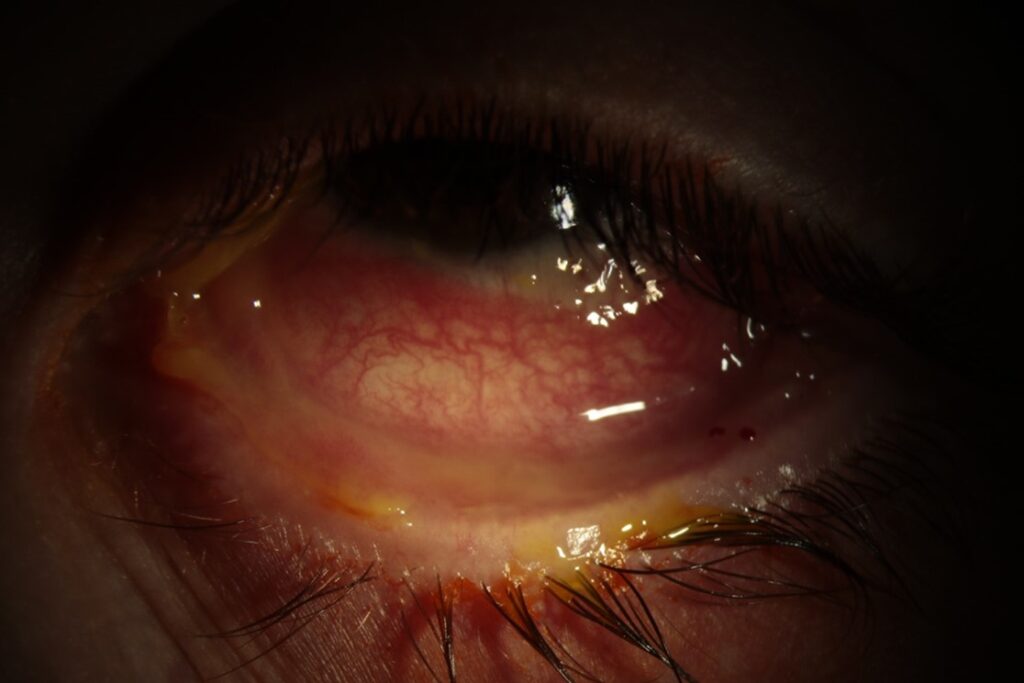
Figure 2. Left eye
A full panel of bloods were taken (FBC, U+E, LFTs, Inflammatory markers). Of note, erythrocyte sedimentation rate (ESR) was 23 mm/hr [normal: 1-13] and C-reactive protein was 20mg/l [normal: <10].
The patient underwent a conjunctival biopsy under general anaesthesia. Two full thickness biopsies were taken from the temporal bulbar conjunctiva of the left eye (the eye with more progressive disease). One was sent for histology (in formalin) and the other was sent for direct immunofluorescence (DIF) (in saline).
Diagnosis & Management
Upon awaiting the biopsy results, the differential diagnosis list was as follows:
- Mucous membrane pemphigoid
- Drug induced mucous membrane pemphigoid
- Pseudo Stevens-Johnson syndrome
- Isolated conjunctival lichen planus
- Ulcerative colitis associated mucous membrane pemphigoid
- Inflammatory bowel disease (IBD)-associated linear IgA disease
- IBD-associated epidermolysis acquisita.
During this time, the patient was admitted for intravenous Methylprednisolone (500mg daily for 3 days) followed by a course of high dose oral steroids. He was commenced on mycophenolate and advised to continue infliximab. This allowed time to establish the diagnosis whilst awaiting the DIF results.
The conjunctival biopsy showed an ulcerated mucosa with a heavy inflammatory infiltrate composed of lymphocytes, plasma cells and polymorphonuclear leucocytes. This was present in a band-like manner at the mucosal-submucosal junction. There was no evidence of granuloma formation or vasculitis. The DIF showed no obvious epidermal lining of IgA, IgG, IgM, C3 and Fibrinogen with strong IgM linear deposition around numerous accessory lacrimal tissues and was therefore inconsistent with mucous membrane pemphigoid. The histology was consistent with Steven Johnson syndrome.
Following this, a more detailed drug history was taken and it became apparent that he had taken a short course of lymecycline 4 months earlier, shortly before the onset of his symptoms. Tetracyclines are a known cause of drug-induced Stevens Johnson syndrome. Unusually, the patient did not have a rash, fever or blistering in any other mucous membranes. The inciting medication had already been stopped.
Sequential amniotic membrane transplants (AMT) (left eye followed by right eye) were performed, one week apart. A modified symblepharon ring was used to fix AMT in place and temporary lateral tarsorrhaphy was made using two 4.0 silk sutures.
Follow-Up
At his last clinic outpatient visit, the visual acuity was 6/18 in the right eye and 6/12 in the left eye. The amniotic membrane transplantations led to complete epithelialisation of both ocular surfaces. Faint stromal haze was present in both corneas from the epithelial defects but this is expected to slowly improve over time. The conjunctiva was also much less oedematous and hyperaemic following the AMT removal. Severe forniceal shortening was still present in both eyes. The patient has been advised to take the following treatment regime:
- Exocin (BD) – both eyes
- Dexafree (QDS) – both eyes
- Serum drops (4-6x/day) – both eyes
- Hylonight (nocte) – both eyes
- Mycophenolic acid/Myfortic (720mg once daily) – orally
- Infliximab (5 mg/kg every 8 weeks) – intravenously
- Prednisolone (30mg daily on a tapering schedule) – orally
- Omeprazole (40mg once daily) – orally
The main concern going forward is the conjunctival fibrosis and how this effects his lid position and corneal epithelial defects and pannus caused by distichiasis and trichiasis. Once the patient, has recovered from the acute inflammation it may be appropriate to consider eyelid surgery to correct this.
Conclusions
- There is a broad spectrum of diseases that may lead to cicatricial conjunctivitis including autoimmune diseases (most commonly ocular cicatricial pemphigoid), medications, atopy, infections, burns and malignancies. The work-up requires serological testing and a biopsy from the perilesional bulbar conjunctiva for histopathology and direct immunofluorescence.
- A lack of any characteristic skin rash or mucus membrane bullae (other than the eyes) does not exclude Stevens Johnson syndrome. This may present with findings isolated to the conjunctiva as demonstrated in this very unusual case. It is possible that the infliximab therapy and low-dose oral prednisolone that he was taking for ulcerative colitis altered the clinical presentation.
- A detailed drug history is essential in all patients with cicatricial conjunctivitis. Although most findings (skin or mucosal) occur within 3 weeks of consuming the drug, there may be a longer lag time from drug intake to Stevens Johnson syndrome development, particularly where the findings were slowly progressive and limited to the eye.
- Maintenance of the ocular surface with preservative free lubricants, topical steroids, topical antibiotics and AMT is essential during the active phase of cicatricial conjunctivitis.
- Early intervention with AMT may prevent corneal scarring, symblepharon or ankyloblepharon.
- Long-term follow-up is required to prevent and address any lid or corneal sequelae that may occur such as lid margin keratinization, distichiasis, trichiasis, entropion, corneal pannus and persistent corneal epithelial defects
Latest Articles


HCP Popup
Are you a healthcare or eye care professional?
The information contained on this website is provided exclusively for healthcare and eye care professionals and is not intended for patients.
Click ‘Yes’ below to confirm that you are a healthcare professional and agree to the terms of use.
If you select ‘No’, you will be redirected to scopeeyecare.com
This will close in 0 seconds