Glaucoma
Introduction
Glaucoma is an eye condition which causes damage to the optic nerve. The official definition is “a group of progressive optic neuropathies characterised by slow, progressive degeneration of retinal ganglion cells and their axons”.1
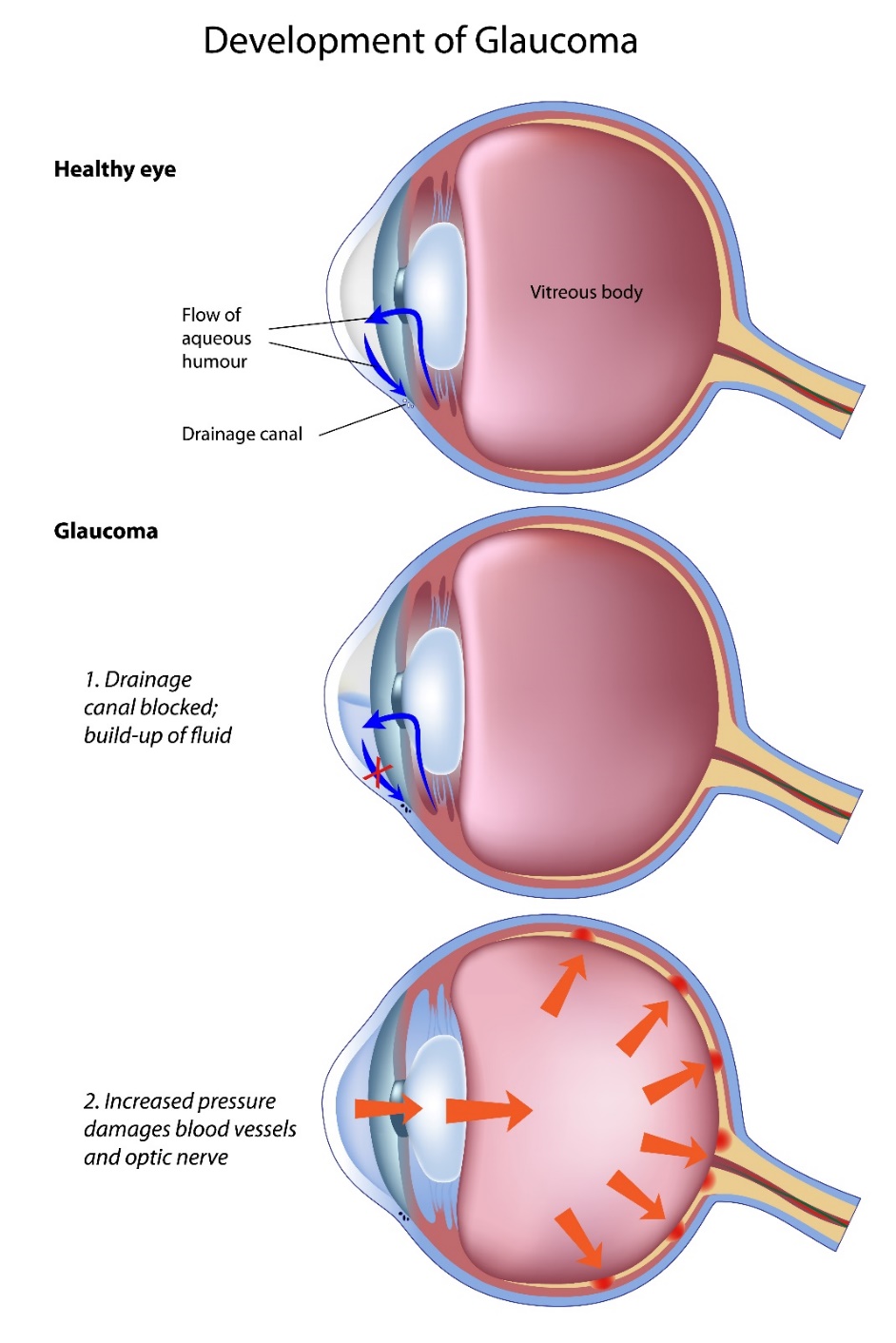
There are different forms of glaucoma and together they are a leading cause of worldwide blindness.2 If glaucoma is undetected, untreated or under-treated, it may lead to a loss of visual function which presents as loss of peripheral vision. Patients may be unaware of this in the early stages. Glaucoma results in characteristic changes in the appearance of the optic nerve head and retinal nerve fibre layer & patterns of visual loss. It can affect one eye or both eyes.
Raised pressure inside the eye (intraocular pressure (IOP) is a major risk factor for development of glaucoma.3 Reducing IOP reduces the rate of progression of glaucoma.4-6 Currently IOP is the only modifiable risk factor and the mainstay of treatment is to lower IOP. Without adequate treatment, glaucoma can progress to visual disability and eventual blindness.7
There are different ways to categorise glaucoma and they all complement each other. The European Glaucoma Society (EGS) classification is based on the mechanism of IOP outflow obstruction.
- Open Angle
- Primary: Primary Open Angle Glaucoma (POAG); POAG suspect, and Ocular Hypertension
- Secondary: Result of ocular disease, Pseudoexfoliation, Pigment Dispersion Syndrome, Neovascular, result of ocular trauma, iatrogenic, extraocular disease
- Angle closure
- Primary: Includes Primary Angle Closure (PAC), Primary Angle Closure Glaucoma (PACG), Acute Angle Closure (AAC)
- Secondary: with pupil block, without pupil block I(anterior pulling; posterior pushing)
- Developmental
- Primary Congenital (birth to 2 years)
- Late onset childhood or early juvenile
- Secondary Childhood
In the USA, glaucoma classification typically follows guidelines established by professional organizations such as the American Academy of Ophthalmology (AAO) and the American Glaucoma Society (AGS). These guidelines provide a framework for categorizing glaucoma based on various factors, including the underlying cause, clinical findings, and disease progression. Here is an overview of the general approach to glaucoma classification:
- Primary Open-Angle Glaucoma (POAG)
- Angle-Closure Glaucoma
- Further classified as acute angle-closure glaucoma (sudden onset) or chronic angle-closure glaucoma (gradual onset).
- Secondary Glaucoma
- Congenital Glaucoma
- Normal-Tension Glaucoma (NTG)
- Classification Based on Disease Severity
- Common classification systems include the AAO Preferred Practice Pattern guidelines8 and the Hodapp-Parrish-Anderson Criteria.9
Classification may also consider risk factors for disease progression, such as age, ethnicity, family history, central corneal thickness, and presence of other ocular or systemic conditions.
Overall, glaucoma classification in the USA aims to provide a standardized framework for diagnosing and managing different types and stages of glaucoma, allowing for personalized treatment plans and improved patient outcomes.
Worldwide the global prevalence of POAG is around 3% and of primary angle closure glaucoma (PACG) is around 0.5%.10
Anatomy of the Eye
The aqueous humour is clear fluid present in the anterior chamber of the eye. It has important functions:
- Contributes to the rigidity and shape of the globe of the eye and maintains IOP
- Adds to ocular refracting power and optical clarity of the eye
- Provides nutrition to the avascular cornea and lens
- Removes metabolic waste-products via onward drainage
- Helps in defense against infection and inflammation
- Has a protective effect against harmful ultraviolet radiation
The aqueous humour is responsible for the maintenance of the IOP which is the only modifiable risk factor for glaucoma and the progression.16
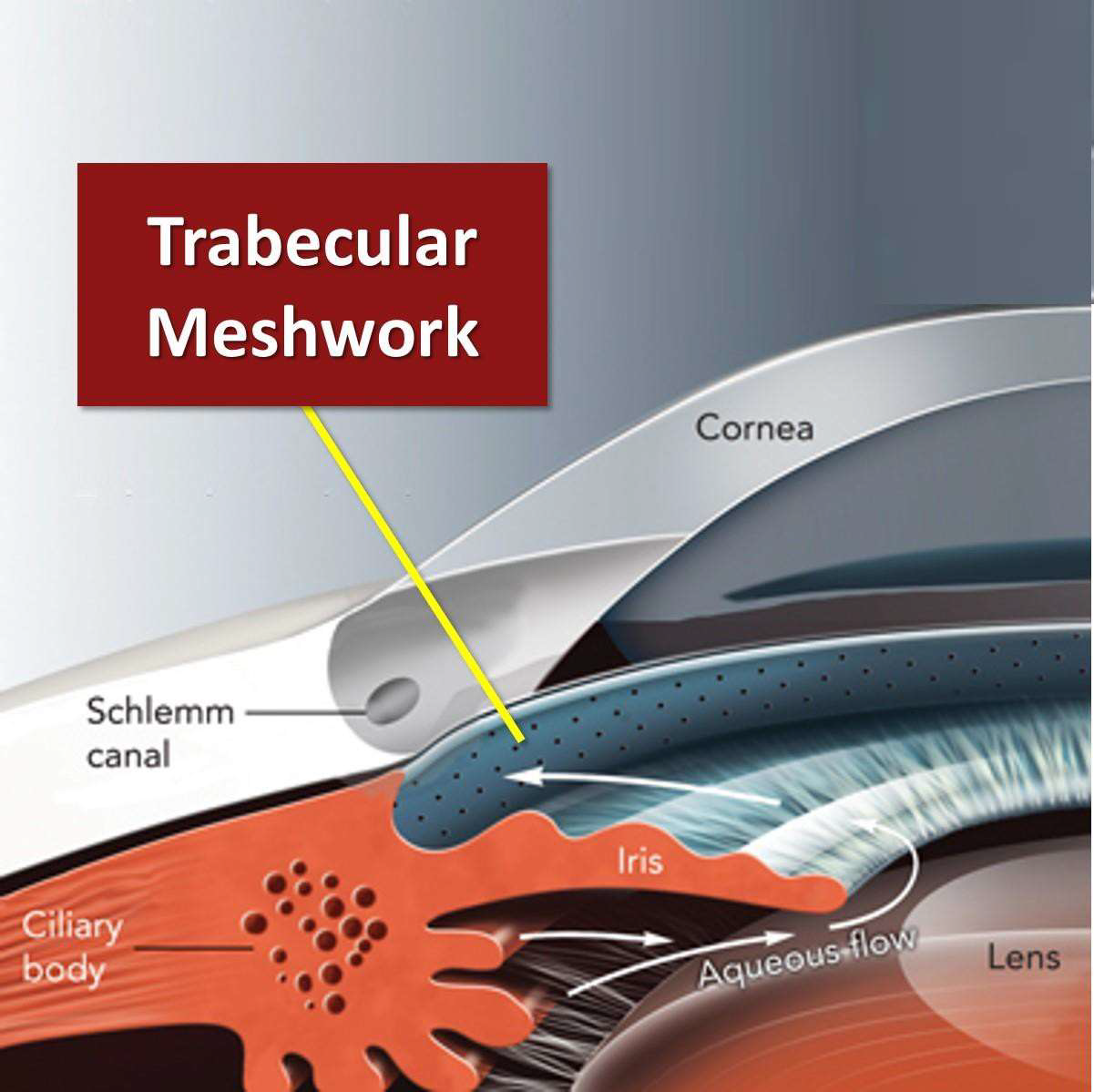
Aqueous humour is produced by the ciliary epithelium of the ciliary body in the posterior chamber. Aqueous flows through posterior chamber and into the anterior chamber. Aqueous then exits the eye via two pathways:
- Trabecular meshwork/canal of Schlemm
- Uveoscleral pathway
The production of the aqueous humour varies during the day. It is highest in the morning, and lowest during the night. The outflow is adjusted throughout the day to account for this. A balance of aqueous inflow and outflow is essential for maintaining ‘normal’ IOP.19
Trabecular Outflow Pathway or Conventional Route
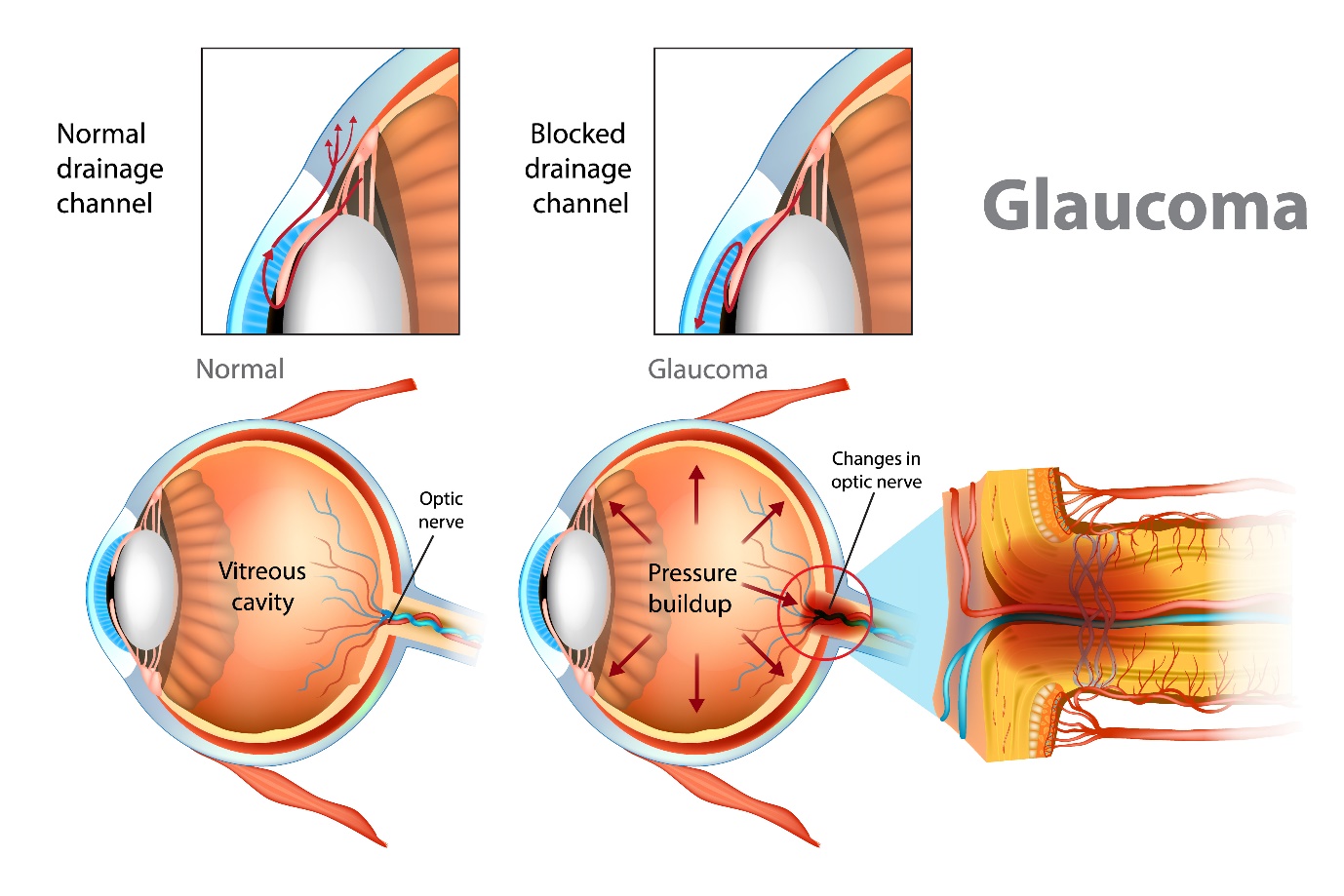
Most of the aqueous humour drains via the trabecular meshwork at the irido-corneal angle. This is an open pore structure which absorbs the aqueous humour. The Canal of Schlemm collects the aqueous and it leaves the eye via the epi-scleral venous network. This pathway accounts for 70-95% of the drainage.20 It is sensitive to IOP changes, and the outflow rate depends on the IOP of the eye.
Uveoscleral Outflow Pathway or Alternative/Unconventional Pathway
This pathway is less well understood. It is thought that approximately 5-30% of aqueous humour leaves the eye via this route. The aqueous enters the ciliary body and passes through it and leaves the eye via veins in the ciliary muscle and drains into the vortex veins. The uveoscleral pathway is not pressure dependent so mostly remains consistent.20
Optic Nerve Head
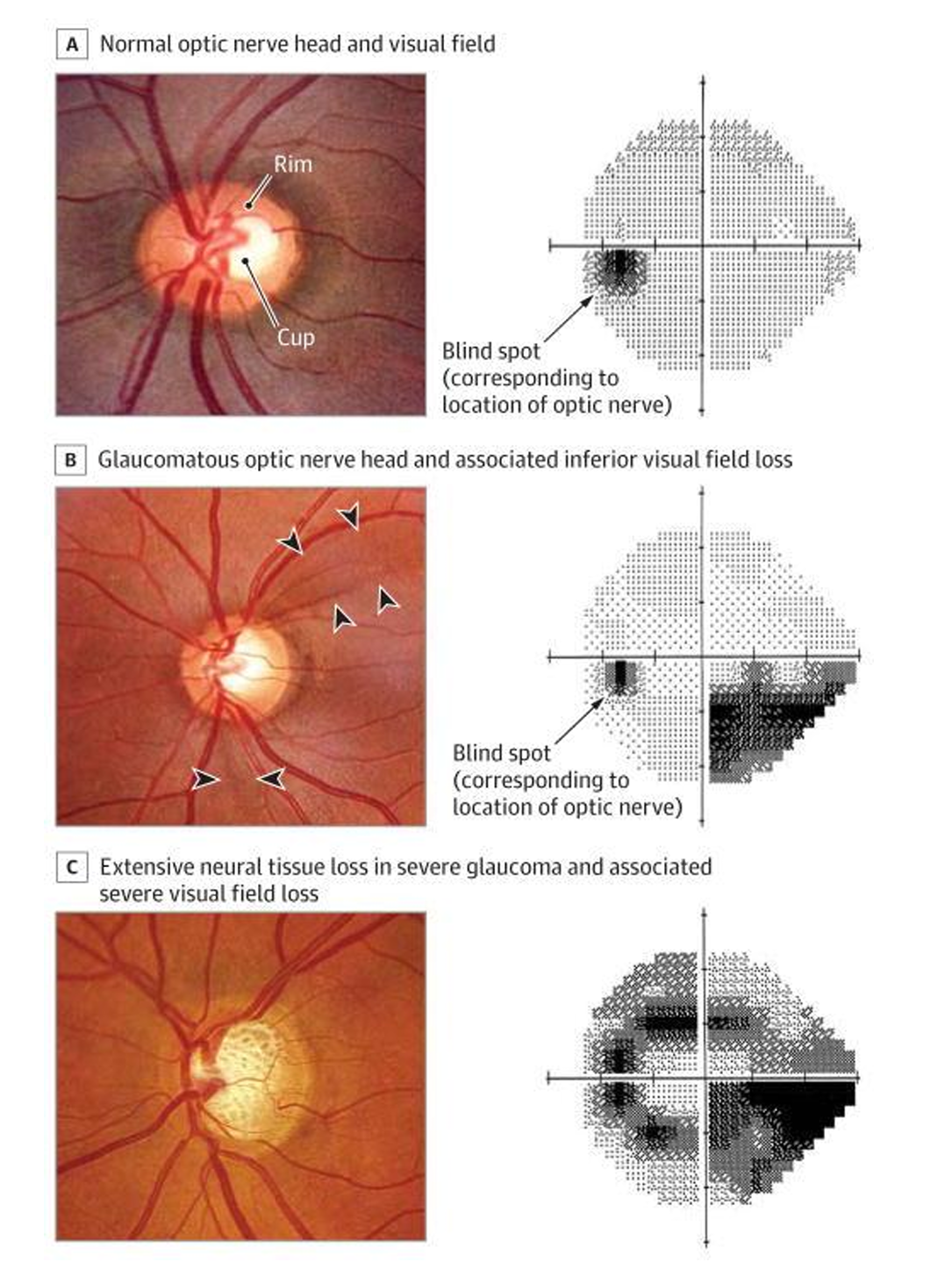
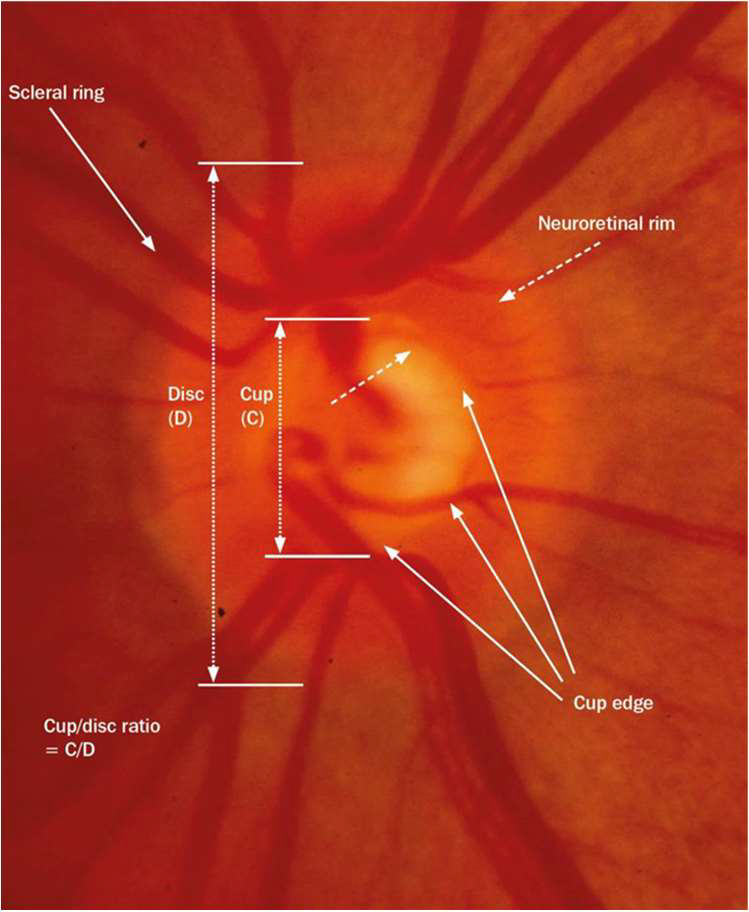
The optic nerve head (ONH) is the second cranial nerve (CN II). It passes all visual information form the eye to the brain. It is the only visible part of the central nervous system. As well as allowing the exit of the retinal ganglion cell axons the optic nerve also allows entry and exit of the retinal blood vessels.21 The retinal ganglion cells are neurons that receive signals from the photoreceptors in the retina. They process the signals and transmit them in axons through the optic nerve to the brain.16 There are 1.2 million individual neurons in the optic nerve. Over time this number decreases with age and this has been estimated to be around 6000 axons per year.
Glaucoma causes damage and death of the retinal ganglion cells at an increased rate. This can lead to progressive impairment of the visual field.16 In glaucoma examination of the optic nerve often reveals characteristic features of glaucomatous optic neuropathy and is an important diagnostic sign.
The features of glaucoma differ depending on the type. The most common can help to distinguish glaucomatous optic neuropathy from other optic neuropathies. The most common features seen in glaucomatous optic neuropathies are:
- Abnormalities of neuroretinal rim
- Abnormalities of peripapillary retinal nerve fibre layer
- Increasing disc excavation (cupping)
- Retinal nerve fibre layer (RNFL) haemorrhages at the disc margin
Causes for Glaucoma
The causes for glaucoma are complex and mostly still under investigation via genetics and testing for treatments.14 Theories of glaucoma pathogenesis:
- Mechanical – The mechanical theory for the cause of glaucoma focuses on the role of IOP. Increased resistance to the outflow of aqueous humour results in an increase in IOP. This leads to mechanical stress and damages the ganglion cells.
- Vascular – The vascular theory is based on poor ocular blood flow (perfusive neuropathy). It is thought there is insufficient blood flow to the eye which can be due to raised IOP or low blood pressure. This causes mild hypoxia (reduced availability of tissue oxygen) and /or ischaemia (reduced tissue nutrients).
- Immune related – This theory suggested that glaucoma may arise from an autoimmune response in the patient. It is thought that mechanical stress leads to the release of neurotoxic factors which then result in ganglion cell death.
Clinical Evaluation
Risk Factors
There are different risk factors for the different types of glaucoma.
Risk factors for POAG:
- Older age. Population studies have shown that POAG increases in prevalence with advancing age.16, 22
- Ocular risk factors
- Elevated IOP22
- Optic nerve head changes
- Thin central corneal thickness (CCT)
- Corneal biomechanics
- Myopia16, 22
- Non-caucasian ethnicity. Population studies have shown that POAG increases in prevalence with ethnicity.23, 24 Glaucoma is the most common cause of blindness amongst Afro-Caribbean ethnicity.14
- Family history of glaucoma. Glaucoma has an estimated heritability of 70%.11 A first degree relative has a 22% risk in their lifetime of developing glaucoma in comparison to 2.3% for non-relatives.14,22
- Vascular risk factors including hypertension and diabetes.22
- Drug induced. This is mainly steroid, (systemic or topical) but can also include drugs such as antihistamines, Parkinson’s medications, botulinum toxin, topiramate, tricyclic antidepressants14 which are commonly prescribed to patients.
Risk Factors for NTG:
- Glaucoma with low IOPs
- More common in women with vascular dysregulation25
- Migraine
- Raynaud’s syndrome
- Older age
- Presence of RNFL haemorrhages at the optic disc
- Thinner central corneal thickness (CCT)
Risk Factors for OHT:
This includes conversion to POAG and is based on the Ocular Hypertension Treatment Study (OHTS)26 and the European Glaucoma Prevention Study (EGPS):27
- Older age
- Higher IOP
- Higher pattern standard deviation in visual fields
- Thinner CCT
Risk Factors for PAC:25
- Increasing age
- Hypermetropia
- Female gender
- Ethnicity
- Diabetes
- Shorter axial length
- Shallow central anterior chamber (AC)
- Large, more anterior lens
To detect and monitor glaucoma, depending on local guidance, it is usual for the clinician to conduct some or all of the following in the examination:8
- Full patient history
- Visual acuity
- Pupil examination
- Slit lamp examination
- IOP measurement
- Gonioscopy
- Optic nerve head examination
- Fundus examination
- Central corneal thickness measurement
- Visual field examination
- ONH, RNFL and macular imaging
Signs
The signs and symptoms of glaucoma can vary depending on the type. Here are the typical signs of the different types of glaucoma although not all may be seen with each type and practitioners are encouraged to conduct a full examination.
- Primary Open-Angle Glaucoma (POAG)
- Elevated Intraocular Pressure (IOP). Although IOP can be within normal ranges in some cases.
- Optic Nerve Cupping. Increased cup-to-disc ratio observed on fundus examination.
- Visual Field Defects: This typically start peripherally and can be detected through visual field testing.
- Retinal Nerve Fiber Layer (RNFL) Thinning. This can be seen on optical coherence tomography (OCT).
- Angle-Closure Glaucoma
- Acute Angle-Closure Glaucoma
- Elevated IOP. This is often significantly higher than normal.
- Corneal Oedema. The cornea is hazy or cloudy due to fluid buildup.
- Shallow Anterior Chamber. There is reduced depth of the anterior chamber of the eye.
- Mid-Dilated Pupil. The pupil may be fixed and mid-dilated, non-reactive to light.
- Conjunctival Hyperaemia. There may be redness of the eye.
- Acute Angle-Closure Glaucoma
- Chronic Angle-Closure Glaucoma
- Elevated IOP. There is a gradual increase in IOP over time.
- Peripheral Anterior Synechiae. The iris may adhere to the trabecular meshwork, and this may be observed on gonioscopy.
- Optic Nerve Damage. This may be similar to POAG, with optic nerve cupping and visual field loss.
- Normal-Tension Glaucoma
- Optic Nerve Cupping. This may be similar to POAG despite normal IOP.
- Visual Field Defects. This may be similar to POAG, and often more localised.
- Retinal Nerve Fiber Layer (RNFL) Thinning. Again this may be similar to POAG.
- Secondary Glaucoma
- Signs Depend on Underlying Cause
- There may be evidence of eye injury, hyphaema, or angle recession.
- Clinicians may observe keratic precipitates, posterior synechiae, and cells/flare in the anterior chamber.
- There may be a history of corticosteroid use leading to elevated IOP.
- Signs Depend on Underlying Cause
- Congenital Glaucoma (in infants and young children)
- This is where there may be an enlarged eyeball.
- Corneal Oedema. There may be a hazy or cloudy cornea.
- Haab’s Striae. There may be breaks in Descemet’s membrane.
- Elevated IOP. This may need to be measured using specialised paediatric tonometry.
- Increased Corneal Diameter. The corneal diameter may be larger than normal.
- Pigmentary Glaucoma
- Pigment Dispersion. Pigment granules may be seen on the corneal endothelium, trabecular meshwork, and iris.
- Krukenberg Spindle. There may be vertical pigment deposition on the corneal endothelium.
- Iris Transillumination Defects. Can be seen on slit-lamp examination.
- Elevated IOP. This is due to blockage of trabecular meshwork by dispersed pigment.
- Pseudoexfoliation Glaucoma
- Exfoliative Material. White, flaky material can be seen on the lens capsule, pupillary margin, and other ocular structures.
- Increased IOP. Due to deposition of exfoliative material in the trabecular meshwork blocking aqueous outflow.
- Zonular Weakness. May lead to lens subluxation.
- Neovascular Glaucoma
- Rubeosis Iridis. New blood vessels may be seen on the iris.
- Neovascularization of the Angle. Abnormal blood vessels in the anterior chamber angle can be observed on gonioscopy.
- Elevated IOP. This is often difficult to control.
- Conjunctival Injection. Redness of the eye.
Each type of glaucoma presents with specific clinical signs that can be identified through a comprehensive eye examination, including tonometry, gonioscopy, slit-lamp examination, fundus examination, visual field testing, and imaging techniques like OCT. Early detection and appropriate management are crucial in preventing vision loss associated with glaucoma.
Symptoms
Symptoms are very dependent on the type of glaucoma a patient has and the severity of the condition – it can vary from asymptomatic to severe pain with acute angle closure glaucoma. Fifty percent of patients do not know they have glaucoma with many people having asymptomatic onset.2
In open-angle glaucoma patients:
- Asymptomatic until an advanced stage- sometimes not affecting both eyes in the same part of the visual field. The overlap would mask the loss.4
- Visual field loss
However, with angle closure glaucoma, there can be:4
- Ocular pain affecting one eye
- Blurred or foggy vision affecting one eye that may last several hours
- Visual field loss
- Conjunctival hyperaemia
- Nausea and vomiting
- Tense, hard globe
Management & Advice
Treatment Paths for Glaucoma
Clinicians are advised to check their local guidance on how to manage patients with suspect or diagnosed types of glaucoma.
The EGS (European Society Guidelines) state that the normal range of intra ocular pressure (IOP) is 10 to 21mmHg (mmHg stands for millimetres of mercury and is the measurement used for eye pressure). In the UK the NICE Guidelines recommend referral if IOP is greater than 24mmHg in the absence of other clinical findings. Bear in mind that OHT is diagnosed as IOP of 21mmHg or greater. This is the case in the USA29 and the UK.
It is estimated that 5 to 10 million Americans with OHT have elevated IOP above 21 mmHg without any evidence of optic nerve damage.
Indications for treatment are often not clear cut but early detection of suspicious changes and prompt treatment can reduce the risk of glaucoma related blindness.
Open Angle Glaucoma Treatment
There are several ways of treating open angle glaucoma. They all involve lowering the IOP. This may be with:
- Prescription eyedrops which either decrease the amount of aqueous humour being produced, or by increasing the rate at which aqueous humour flows out from the eye, or a combination of both.
- Prostaglandin Analogues
- Beta-Adrenoreceptor Antagonists (b-Blockers)
- Alpha-Adrenoreceptor Agonists (α-Agonists)
- Carbonic Anhydrase Inhibitors
- Cholinergic Agonist
- Rho Kinase Inhibitor (USA and privately in the UK)
- Nitric Oxides (USA)
- Laser treatment to increase the rate at which aqueous humour drains away. This is often as effective as a single medication for lowering IOP.14
- Selective Laser Trabeculoplasty (SLT) Can be repeated
- Argon Laser Trabeculoplasty (ALT)
- Surgery to increase the rate at which aqueous humour drains away e.g.
- Trabeculectomy
- Aqueous Shunt Surgery
- Minimally Invasive Glaucoma Surgery (MIGS)
The type of treatment a patient is offered depends on local guidance but also the severity of the disease. For example in the UK NICE now recommends that all newly diagnosed glaucoma patients are offered SLT laser treatment as a first option.
In the USA the American Optometric Association is reviewing its 2010 guidance but currently states topical treatment is the first option. The American Academy of Ophthalmology also states “Medical therapy is presently the most common initial intervention to lower IOP.”
Primary Angle Closure Treatment
In the UK the Royal College of Ophthalmologists now recommend that clear lens extraction (CLE) is superior to laser peripheral iridotomy in PACG. In PAC in patient with IOP up to 30mmHg PI is still recommended, and in patients with IOP greater than 30mmHg CLE is recommended. This is based on the EAGLE study. [30] for the following reasons:
- Better disease control
- Cost effective
- Better quality of life
The American Academy of Ophthalmology guidance can be found here31 and it discusses medication, laser therapy and surgery, while recommending that “cataract (or clear lens) extraction could be considered for the management of PAC and PACG” by the clinician.
Acute Angle Closure Treatment
This is a medical emergency and requires prompt treatment.
- Medical management includes:
- Miotics
- e.g. Pilocarpine 2-4%
- Systemic agents
- e.g. PO. Acetazolamide
- Topical ocular hypotensives
- e.g. Timolol
- e.g. Dorzolamide
- e.g. Brimonidine
- Surgical treatment
- Laser peripheral iridotomy once medical management has been successful
- Trabeculectomy
- Phacoemulsification
- Cylclodiode laser in case that are unresponsive to medical therapy
- Miotics
This article serves as an overview of the condition and treatment options. It does not serve as a clinical guidance. Eyecare provider guidelines should be used when managing patients.
This article serves as an overview of the condition and treatment options. It does not serve as a clinical guidance. Eyecare provider guidelines should be used when managing patients.